Riassunto
Nannodastiidae e Coelopidae: due famiglie di ditteri acalitteri nuove per l’Italia, con descrizione di una nuova specie di Azora-stia Frey (Nannodastiidae) (Diptera: Brachycera)
Sono segnalate per la prima volta in Italia specie di ditteri delle famiglie Nannodastiidae e Coe-lopidae. Una nuova specie del genere Azorastia Frey (Nannodastiidae) viene descritta e i genitali del maschio illustrati.
Abstract
The families Nannodastiidae and Coelopidae (Diptera) are recorded for the first time from Italy. A new species of Azorastia Frey is described and the male terminalia figured.
Introduction
Nannodastiidae and Coelopidae are two phyletically unrelated families of true flies inhabiting the oceanic and Mediterranean supralittoral belts. These two families are recorded here for the first time from Italy (see further) and a new species of the former family is described. The site of Balzi Rossi (Ventimiglia, Liguria, NW Italy), where the two families of flies were collected by the Vene-tian naturalist G. Rallo, had been previously reported in the literature for the first Italian record of the family Xenasteiidae (Vanin, 2003). Due to their min-ute size, the Nannodastiidae and Xenasteiidae are very often overlooked by entomologists other than dipterists and even by fly collectors that, in many cases, neglect their preparation and identification at family level. Differently, the medium-sized Coelopidae are very easily collected and studied. Indeed, populations of Coelopa Meigen, 1830 are often very abundant in individuals, and may reach exceedingly high levels when there is plenty of stranded kelp (brown algae). But, the coelopid flies are represented on the Mediterranean coasts by only one uncommon species belonging to the monotypic genus Mala-comyia Haliday in Westwood, 1840, which occurs mainly on stony seashoresrather than on sandy beaches.
Materials and methods
The two specimens examined here are double mounted, micropinned in a plas-tic block. Study and illustrations of the new species required the use of dis-secting and compound microscopes, the latter used in particular for perusal of the genitalic structures. Fine-tipped tweezers and micropins were used to remove and dissect the abdomen, which was macerated in a boiling, potassium hydroxide solution. The abdomen was dipped in a 20% acetic acid solution for about three minutes to fully neutralize the action of caustic potash, and then rinsed in distilled water. The entire postabdomen was then transferred to glycerine for observation. When necessary for proper orientation, the structure was transferred from glycerine to glycerine jelly. The glycerine jelly was heated, and the piece appropriately oriented. After cooling, the piece embedded in glycerine jelly became immobilized. Abdomen and genitalia were in this way studied, photographed, and drawn, and finally placed in a small plastic tube filled with glycerine, and pinned below the specimen from which the structure had been removed. The descriptive terminology chiefly follows the Manual of Central American Diptera (CummingandWood, 2009). Holotype label data isquoted verbatim, i.e. without interpretation; a slash (/) indicates the end of a line of print or handwriting, two slashes (//) indicate the end of a label and the beginning of another. Significant supplementary or qualifying information is given in brackets.
Taxonomic account
Family Nannodastiidae Papp, 1980 [as a subfamily of Ephydridae] (Nannodastiid beach flies)
A family of minute true flies, sometimes less than 1 mm long. These tiny flies occur on widely separated coastal areas of the Mediterranean Sea as well as on seashores of the Atlantic and Indopacific Oceans. Because of their minute size, they are very rarely collected, prepared and studied. The two closely related genera in the family, Azorastia Frey, 1945 and Nannodastia Hendel, 1930, were previously placed into different families (Asteiidae, Chyromyidae, Ephydridae), until Carles-Tolrá (1994) accorded to them a separate familial status (Nanno-dastiidae) based on Papp’s (1980) designation of the Nannodastiinae as a new subfamily within Ephydridae. According to Papp and Mathis (1998) and Ma-this (2010), however, the two genera comprising this family remain enigmatic, with unknown familial affiliations within the Schizophora Acalyptratae (super-families Opomyzoidea/Carnoidea). The biology of these flies is unknown, but the larvae are probably microbial grazers and saprophagous in shoreline debris. Papp and Mathis (2001) provided an exhaustive review of the family. The genus Azorastia is known so far with only four species from the western Palaearc-tic seacoasts, including the new species described in the present work (NB: Azorastia gemmae Carles-Tolrá, 1994 was described from the Balearic Islandsand not from Estonia as erroneously reported in the FaEu portal (last accessed February 5, 2016) and no additional records have been reported so far in the literature). The family Nannodastiidae is formally recorded here for the first time from Italy.
Azorastia ralloi sp. nov.
(Figs. 1-3)
Diagnosis
External features strikingly similar to congeners, agreeing with diagnoses and descriptions of those species, except for the distinctive male terminalia (figs. 1-3).
Description
A minute dark brown to extensively black fly; body length 1.26 mm; wing length 1.27 mm; wing width 0.55 mm.
Head. Distinctly higher than long; frons and occiput black, the latter stronglydepressed; frontal triangle large, chloropid-like, almost reaching anterior mar-gin of frons; medial vertical and lateral vertical setae subequal in length; ocellar setae of moderate size, inserted laterad of anterior ocellus, behind them a pair of minute setae inserted immediately behind posterior ocelli; postocellar seta (= postvertical of authors) lacking; 3 reclinate frontorbital setae, anterior seta slightly shorter, inserted about at level of lunula; antenna dark brown (pedicel) to black (basal flagellomere), the latter very long haired; arista black, short, micropubescent; dorsal face depressed, apparently weakly sclerotized medially, ventral face slightly protrudent; clypeus small; 4 strong, upcurved, black peri-stomal setae; gena pale yellow, very narrow (ratio: vertical height of eye / height of gena = 8.69).
Thorax. Dark brown to black. 0+1 dorsocentral seta; 1 postalar seta; 2 scutellarsetae, apical seta distinctly longer; 1 row of dorsocentral setulae; 1 intra-alar row of setulae; acrostichal setulae arranged in two regular rows, well discernible on the anterior half of scutum; postpronotal setae lacking; 2 strong notopleural setae, posterior one inserted at much higher level then anterior seta; 5 anepis-ternal setae, 2 of which upcurved; 1 katepisternal seta. Legs yellowish, evenly setulose, without major setae. Wing hyaline; second / third costal sections (0.41 0.50 mm) = 0.82; distance between crossveins r-m and dm-cu / apical section of vein CuA1 = 1.0; costal vein with humeral and subcostal breaks; subcosta incomplete, rudimentary; costa distinctly extended beyond end of vein R4+5; costal vein with both larger and smaller setae, the former widely spaced; vein M1 thin, pale, apically reaching the wing margin as a faint fold; crossvein r-m very small, located approximately in vertical correspondence with the anal lobe; crossvein dm-cu present; anal cell (posterior cubital cell) and anal vein lacking; alula lacking (or strongly reduced). Halter black, with a remarkably big knob (capitulum).
Abdomen (figs. 1-3). Entirely black, with inconspicuous, marginal and dorsalsetae on each tergite; sternites bearing scattered, minute setulae; cuticle of ter-gites microscopically wrinkled transversally; tergites 1 and 2 not observed be-fore dissection (probably separated completely as congeners); last visible tergite 6; a very narrow, strongly sclerotized sclerite present in membrane between tergite 6 and epandrium, forming a horseshoe-shaped half ring (syntergoster-nite 7+8); male terminalia symmetrical; surstylus simple, widely lobe-shaped in lateral view (tapered and acutely pointed apically in posterior view), partially fused with epandrium; external side of surstylus with 3 distinct, longitudinal, dark folds; posterior margin of the right surstylus showing a minute notch (fig. 3) probably due to an accident occurred when the fly was still alive; cercus with some obvious, marginal tubercles, each bearing a long seta; internal parts of male genitalia forming a very characteristic lever structure (typical for the fam-ily) (fig. 1); hypandrium strongly elongate, fused anteriorly with phallapodeme; phallapodeme fused with basiphallus; gonopods or parameres lacking; a sube-pandrial sclerite (or sternite 10) (according to Papp and Mathis, 1998) connects the posterior ends of hypandrium and cerci; phallus very long and slender.
Type material
The holotype is labelled: [handwritten white label] “[Italy] Ventimiglia – IM [province of Imperia] / Balzi Rossi 5.8.80 [5.viii.1980] [43°47’01.20” N – 7°32’12.53” E] / in grotta sul mare [in a cave facing the sea]” / leg. G.P. Rallo” // [printed red label] “HOLOTYPUS / Azorastia ralloisp. nov. ♂ [handwrit-ten male symbol] / L. Munari – 2016”. The holotype (only known specimen) is in moderately good condition. Left wing and left hind leg, detached by ac-cident, were mounted permanently in polyvinyl-lactophenol on a celluloid slide pinned below the specimen. Right wing lost. The abdomen was detached, clari-fied and stored in a plastic microvial pinned below the specimen. The holotype is deposited in the Diptera collection of the Natural History Museum, Venice (Type inventory: MSNVE #5156).
Female. Unknown.
Larva and puparium. Unknown.
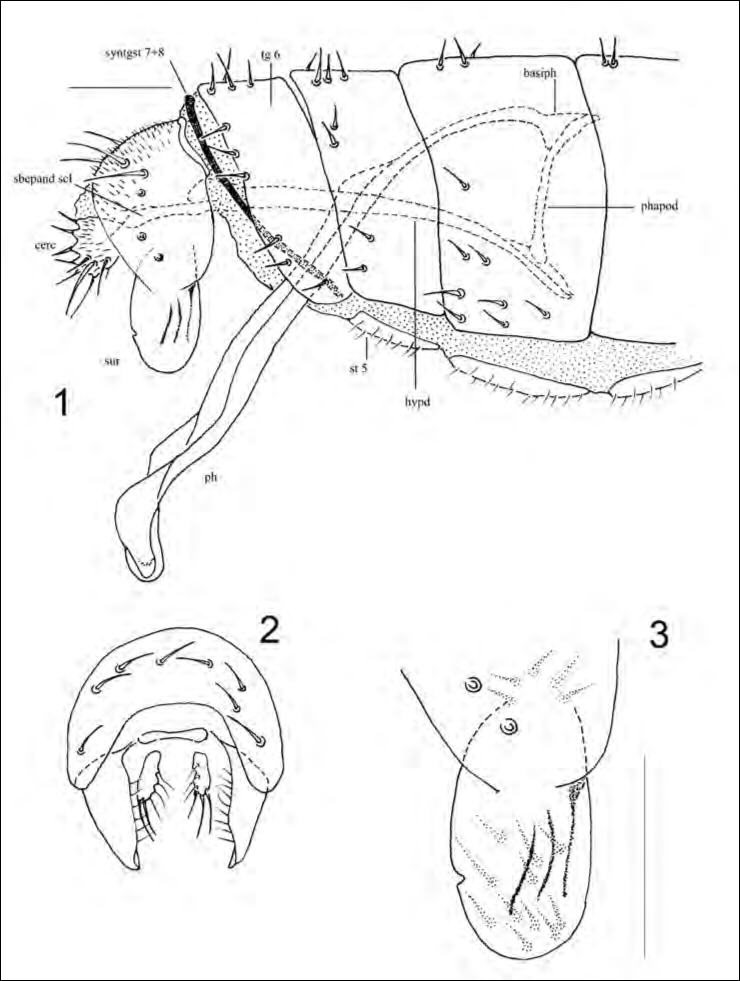
Abbreviations: basiph = basiphallus; cerc = cercus; hypd = hypandrium; ph = phallus; phapod = phallapodeme; sbepand scl = subepandrial sclerite; st = sternite; sur = surstylus; syntgst = syntergosternite; tg = tergite.
Biology. Unknown. Probably a saprophagous, coastal fly as congeners. Distribution. Northwest Italy (West Liguria).
Etymology. The species epithet, ralloi, is a genitive patronym to recognize the contribution of Giampaolo Rallo, who collected the holotype. Remarks
Azorastia ralloi sp. nov. is closely related to congeners, especially to A. minutis-sima Frey, 1945 and A. mediterranea Papp, 1980, but differs in the shape ofthe male surstylus (figs. 1-3). The site where the new species was collected is characterised by a small sea cave lit by daylight, about ten meters away from the shoreline. The specimen was netted by sweeping over a stony/sandy substrate partly soiled with guano of pallid swift (Aves: Apus pallidus (Shelley, 1870)) and possibly (not observed) of bats. The site is today heavily anthropized during the bathing season, and a beach resort has been built in close proximity to the cave entrance (G. Rallo, Jan. 2016, pers. comm.).
Key to species of the genus Azorastia Frey
(modified and updated after Papp and Mathis, 2001)
1. Male surstylus very long, length over twice width, narrow basally, thereafter becoming wider to apical one third, then curved posteriorly and pointed (figs. 1-2, 4 of Carles-Tolrá, 1994)………………………… A. gemmae Carles-Tolrá
- Male surstylus moderately long to short, length less than twice width, not narrowed basally ………………………… 2
2. Ratio of intercrossvein section of vein M1 / terminal section of vein CuA1 usually 1.00 ………………………… 3
- Ratio of intercrossvein section of vein M1 / terminal section of vein CuA1 1.20-1.30 ………………………… A. minutissima Frey
3. Male surstylus short, less long than half of dorsoventral length of epandrium, anterior edge more or less protuberant in profile (figs. 5-7 of Papp and Ma-this, 2001) ………………………… A. mediterranea L. Papp
- Male surstylus distinctly longer, about as long as half of dorsoventral length of epandrium, regularly lobe-shaped in profile, anterior edge not protuberant, quite similar to posterior edge (figs. 1, 3) …………………. A. ralloi sp. nov.
Family Coelopidae Hendel, 1910 [as a subfamily of Muscidae s.l.] (Seaweed or kelp flies)
All species of the family Coelopidae are thalassobiontic, seaweed flies that abound in the piles of brown algae stranded in the supralitoral zone of most temperate oceanic seashores. This family is made up mainly of medium-sized, flattened, dark flies. The family includes 29 valid described species in 13 gen-era (McAlpine, 1991, 1998; Mathis, 2010; Mathis and McAlpine, 2011) with a worldwide distribution. It is divided into two subfamilies, Lopinae, which includes only one species from Australia, and the relatively diverse subfamily Coelopinae (in turn divided into a dozen genera, with more than a third of the species in the genus Coelopa). Coelopidae are in the superfamily Sciomyzoidea and are thought to be most closely related to the Helcomyzidae (McAlpine, 1998; Mathis and McAlpine, 2011), perhaps as their sister group (McAlpine, 1991). Malacomyia is a monotypic genus distributed in Europe and Mediter-ranean seacoasts, westwards reaching the Macaronesian province.
Malacomyia sciomyzina (Haliday, 1833)
Material examined: Italy, Ventimiglia (province of Imperia), Balzi Rossi
(43°47’01.20” N – 7°32’12.53” E), in grotta sul mare [in a cave facing the sea],
5.viii.1980, G.P. Rallo leg..
Remarks
Rondani (1866) described a subspecies meridionalis from Malta (“specimen utri-usque sexus ad littora Melitae capta a Doct. Schembri possideo”). In the checklistof the Italian fauna, Munari and Rivosecchi (1995) regarded Rondani’s subspe-cies as a valid taxon and placed it into the family Dryomyzidae. Considering the close proximity of Malta to the Sicilian coast, those authors assumed the occurrence of this species in that large Italian island to be likely, but not certain
Indeed, they marked the abbreviation “Si” (= Sicily) with a question mark. However, Mathis and McAlpine (2011: 194) reported Italy in the distribution of this species. In all probability, they misunderstood Munari and Rivosecchi’s (1995) doubtful citation of this species from Sicily and, conversely, they consid-ered that record as certain for Italy.
M. sciomyzina is formally recorded here (with certainty) for the first time fromItaly.
Acknowledgements
I wish to thank the following colleagues for their assistance and useful informa-tion: M. Carles-Tolrá (Barcelona, Spain), R. Maier (Singapore), L. Papp (Bu-dapest, Hungary), and G. Rallo (Venice, Italy). Particular thanks are also due to Prof. A. Minelli (Padova, Italy) for his critical reading of a first draft of this article.
References
Carles-Tolrá M. (1994) – Azorastia gemmae: a new nannodastiid species from the Isle of Ibiza (Spain, Balearic Isles) (Insecta: Diptera: Nannodastiidae). Reichenbachia, 30(34): 199-202.
Cumming J.M. and D.M. Wood (2009) – Adult morphology and terminology. Pages 9-50. In: Brown B.V. et al. (editors), Manual of Central American Diptera: Volume 1. NRC Research Press, Ottawa, Ontario, Canada. 714 pages.
Frey R. (1945) – Tiergeographische Studien über die Dipteren der Azoren. Societas Scientiarum Fennica. Commentationes Biologicae.8(10): 1-114.
Hendel F. (1910) – Über acalyptrate Musciden. Wiener entomologische Zeitung, 29:101-127, 1 pl.
Hendel F. (1930) – Eine neue interessante Ephydridengattung (Dipt.). Konowia, 9(1): 66-70.
Mathis (2010) – Nannodastiidae (Nannodastiid beach flies) 101. Pages 1235-1239. In: Brown B.V. et al. (editors), Manual of Central American Diptera: Volume 2. NRC Research Press, Ottawa, Ontario, Canada. 728 pages.
Mathis W.N. and D.K. McAlpine (2011) – A catalog and conspectus on the family Coelopidae (Diptera: Schizophora). In: Brake I. and Thompson F.C. (editors), Con-tributions to the Systema Dipterorum (Insecta: Diptera). Myia,12: 171-205, PensoftPublishers & North America Dipterists Society, Sofia, Moscow & Washington D.C.. McAlpine D.K. (1991) – Review of the Australian kelp flies (Diptera: Coelopidae). Systematic entomology,16: 29-84.
McAlpine D.K. (1998) – 3.31. Family Coelopidae. Pages 335-340. In: Papp L. and Dar-vas B. (editors), Contributions to a Manual of Palaearctic Diptera: Volume 3. Science Herald, Budapest, 880 pages.
Munari L. and L. Rivosecchi (1995) – Diptera Sciomyzoidea. In: Minelli A., Ruffo S. and La Posta S. (editors), Checklist delle specie della fauna italiana, 74: 1-7.
Papp L. (1980) – New taxa of the acalyptrate flies (Diptera: Tunisimyiidae fam. n., Risidae, Ephydridae: Nannodastiinae subfam. n.). Acta Zoologica Academiae Scien-tiarum Hungaricae,26(4): 415-431.
Papp L. and W.N. Mathis (1998) – 3.27. Family Nannodastiidae. Pages 309-314. In: Papp L. and Darvas B. (editors), Contributions to a Manual of Palaearctic Diptera: Volume 3. Science Herald, Budapest, 880 pages.
Papp L. and W.N. Mathis (2001) – A review of the family Nannodastiidae (Diptera). Proceedings of the Entomological Society of Washington,103(2): 337-348.
Rondani C. (1866) – Scatophaginae Italicae collectae distinctae et in ordinem disposi-tae. In: Dipterologiae Italicae Prodromi. Pars VII, Fasc. I., Stirps XVIII, Atti Società italiana di scienze naturali,10: 1-52.
Vanin S. (2003) – Xenasteiidae: a dipterous family new to Italy. Bollettino del Museo civico di Storia naturale di Venezia,54: 91-93.
