Riassunto
Ritrovamento e ridescrizione dell’olotipo di Stephanus (Di-stephanus) [sic!] athesinus Biegeleben, 1929, con commentosulla sua identità e nuovi dati corologici su Megischus anomalipes (Förster, 1855) (Hymenoptera, Stephanidae)
Viene segnalato il ritrovamento dell’olotipo di Stephanus (Distephanus) [sic!] athesinus Biegele-ben, 1929, conservato presso il Museo di Storia Naturale di Venezia (Italia), del quale si fornisco-no una ridescrizione ed un commento sulla sua identità tassonomica. Viene pertanto confermata la sinonimia con Megischus anomalipes (Förster, 1855). Si riportano nuovi dati sulla distribuzio-ne: viene segnalata per la prima volta per le regioni italiane del Veneto, Emilia-Romagna, Toscana e Basilicata e risulta nuova per la Grecia.
Abstract
The rediscovery of the holotype of Stephanus (Distephanus) [sic!] athesinus Biegeleben, 1929, preserved in the collections of the Museo di Storia Naturale, Venice (Italy), is recorded together with a redescription and a comment on its taxonomic identity. Therefore, its synonymy with Megischusanomalipes (Förster, 1855) is confirmed. New distributional data for M. anomalipes is provided:it is recorded for the first time from the Italian regions of Veneto, Emilia-Romagna, Tuscany and Basilicata, and newly recorded from Greece.
Introduction
Stephanidae Leach, 1815 is a cosmopolitan family of Hymenoptera mainly occurring in tropical and subtropical areas, with 346 described species, including fossils (Aguiar, 2004, 2005, 2006; van Achterberg & Yang, 2004; Aguiar & Jennings, 2005; van Achterberg & Quicke, 2006; Aguiar et al., 2010; Hong et al., 2010;Hong&Xu, 2011;Tan et al., 2015). Stephanids are usually medium- tolarge-sized parasitoid wasps, reaching 100 mm in length (including ovipositor) in the genera Megischus Brullè, 1846 and Profoenatopus van Achterberg, 2002 (Aguiar, 2005). They can be easily recognized by the presence of a conspicu-ous ‘crown’ or ‘corona’ on the head, consisting of five more or less developed tooth-like processes on the frons, a very slender body, the pronotum elongate and sub-conical, the hind legs highly modified, with all segments swollen to widened, the hind femur bearing large ventral tooth-like processes. Stephanids are considered to be rare or extremely localized, but this is probably due to their cryptic colour pattern and behavior, with adults preferring walking up and down close to tree bark (Turrisi, personal observation). This fact is deeply reflected in the taxonomic knowledge of the family, with some 95% of described species based on a single specimen (Hong et al., 2011).
The biology of Stephanidae is poorly known. They can be found near dead tree trunks or branches for about one year inhabited by beetle larvae and not yet infested by fungi (van Achterberg, 2002). They are idiobiont ectoparasitoid, feeding on wood boring larvae (Taylor, 1967). Their hosts are coleopterous larvae, mainly of Buprestidae (e.g., Townes, 1949; Chao, 1964; Pagliano, 1986; Braza; 1989; Turrisi, 2002) and Cerambycidae (e.g., Blüthgen, 1953; Völlger, 1994; Visitpanich, 1994; Turrisi, 2002), but there are also records of other fami-lies of Coleoptera, as well as for Hymenoptera Siricidae and solitary Apoidea as possible hosts (Aguiar, 2005; Hong et al., 2011). In order to detect their hosts, Broad & Quicke (2000) and Vilhelmsen et al. (2001) proved that many Hyme-noptera are able to produce vibration signals by tapering the substrate with their antennae. The echoes are detected by an enlarged subgenual organ of the legs. By dissecting the hind tibia, van Achterberg (2002) found part of a supposed subgenual organ, although roughly identified and illustrated. Stephanid wasps seem to drum during the search for hosts (Rodd, 1951), but females do not have modified antennal tips for beating the substrate (Vilhelmsen et al., 2008). Vilhelmsen et al. (2008) described the subgenual organ of stephanids in detail, suggesting that they are able to use passive vibration detection, monitoring sound produced by potential hosts.
In the present paper, we clarify and confirm the synonymy of Stephanus (Dis-tephanus) [sic!] athesinus (Biegeleben, 1929) with Megischus anomalipes (Förster,1855) through the redescription of the rediscovered holotype. We also update the Italian distribution of Megischus anomalipes and provide new records from Europe and NW Asia.
Taxonomic situation of the European Stephanidae
Only two species of Stephanidae, namely Stephanus serrator (Fabricius, 1798) and Megischus anomalipes (Förster, 1855), are known from Europe (Madl, 2013). The former species is known from Central and South Europe, except Portugal, Albania and Greece (van Achterberg, 2002). In Italy, the species occurs all over the mainland, including Sicily and Sardinia (Pagliano, 1986; Turrisi, 2002). Conversely, Megischus anomalipes shows a fragmentary distribu-tion in South Europe and North-West Asia (Turrisi, 2002; van Achterberg, 2002, Madl, 2013).
Megischus anomalipes was first described from Hungary as Stephanus anomali-pes. Sichel(1860) described Bothrioceros europaeus (junior subjective synonymof M. anomalipes) based on a single female from Sicily. Later, Sichel (1865) transferred the species under the genus Megischus, and Schletterer (1889) up-dated the distribution of the species. Biegeleben (1929) described Stephanus (Distephanus) [sic!] athesinus (currently a junior synonym of M. anomalipes)based on a female from Bolzano, but the holotype was not located subsequently and thus believed to be lost. van Achterberg (2002) redescribed Megischus anomalipes on a single female from France, andTurrisi(2002) provided thefirst description of the male.
Materials and methods
The material includes specimens from the Museo di Storia Naturale di Venezia (MSNVE), and Dal Pos D. and Turrisi G.F. private collections. Holotype data label are quoted verbatim, i.e. without interpretation; a slash (/) indicates the end of a line, two slashes (//) mean the beginning of another label.
A dissecting stereomicroscope (OPTIKA SZM-2) was used for observation and study. Photographs were taken by a Canon Eos 600D, lens Canon MP-E 65mm f/2.8 1-5x Macro and Sigma 105mm f/2.8 Macro DG OS HSM, using Combine ZP for the stacking (Hadley, 2008). The distribution map was produced by using QGIS 2.14.3 Essen. Morphological terms follow van Achterberg (2002).
Acronyms for museums as repositories of primary types and other collections examined:
- HNHM Hungarian Natural History Museum, Budapest, Hungary
- MNHN Muséum National d’Histoire Naturelle, Paris, France
- MSNVE Museo di Storia Naturale di Venezia, Venice, Italy
- DPDC Dal Pos Davide, private collection, Ponte della Priula (Treviso), Italy
- TGFC Turrisi Giuseppe Fabrizio, private collection, Pedara (Catania), Italy
Key to species of the European Stephanidae
- Femal…………………………………………………………………………2
- Males……………………….…………………………………………………3
- Ventral margin of hind femur with 3 tooth-like processes (Fig. 6); distal half of hind tibia swollen (Fig. 6); hind tarsus 5-segmented; 1st-3rd tergites of metasoma reddish-brown, 4th tergite entirely black to reddish brown at base, tergites 5-8 entirely black (Fig. 5)
……………………………………………………………………. Stephanus serrator
- Ventral margin of hind femur with 2 tooth-like processes (Fig. 5); distal two thirds of hind tibia swollen (Fig. 5); hind tarsus 3-segmented; 1st tergite of metasoma extensively reddish brown, 2nd tergite red apically, otherwise black, tergites 3-8 entirely black (Fig. 1)
……………………………………………………………………. Megischus anomalipes
- Ventral margin of hind femur with 3 tooth-like processes; distal half of hind tibia swollen; proximal median part of mid and hind basitarsi ivory; distal margin of 3rd hind tarsomere straight; 1st and 2nd tergites of metasoma ex-tensively reddish-brown, 3rd tergite usually reddish-brown at base and then black or entirely red, 4th tergite entirely black to reddish-brown at base, tergites 5-8 entirely black; paramere, in lateral view, with regularly rounded distal margin
……………………………………………………………………. Stephanus serrator
- Ventral margin of hind femur with 2 tooth-like processes ; distal two thirds of hind tibia swollen; proximal half of mid and hind basitarsi ivory; distal margin of 3rd hind tarsomere sinuous; 1st tergite of metasoma extensively reddish-brown, tergites 2-8 entirely black; paramere, in lateral view, with pointed distal margin
…………………………………………………………………….Megischus anomalipes
Taxonomic account
Genus Megischus Brullé, 1846
Megischus Brullé, 1846: 537. Type species: Stephanus furcatus LePeletier & Ser-ville, 1825 [subsequent designation by Viereck, 1914]
Bothrioceros Sichel, 1860: 759. Type species: Bothrioceros europaeus Sichel, 1860[subsequent designation by Aguiar, 2004]
The genus comprises 83 species worldwide (Aguiar, 2004, 2006; van Achter-berg & Yang, 2004; van Achterberg & Quicke, 2006; Hong et al., 2010), three of which from the Palaearctic Region: Megischus anomalipes (Förster, 1855), Megischus ptosimae Chao, 1964 and Megischus aplicatus Hong, van Achterberg,Xu, 2010. Megischus gigas (Schletterer, 1899) occurs in Iran (Masnadi-Yazdinejad Lotfalizadeh, 2009), and has been transferred in the genus Afromegischus van Achterberg, 2002 by Aguiar (2004). In Italy, only one species is recorded for the genus (Scaramozzino, 1995; Turrisi, 2002).
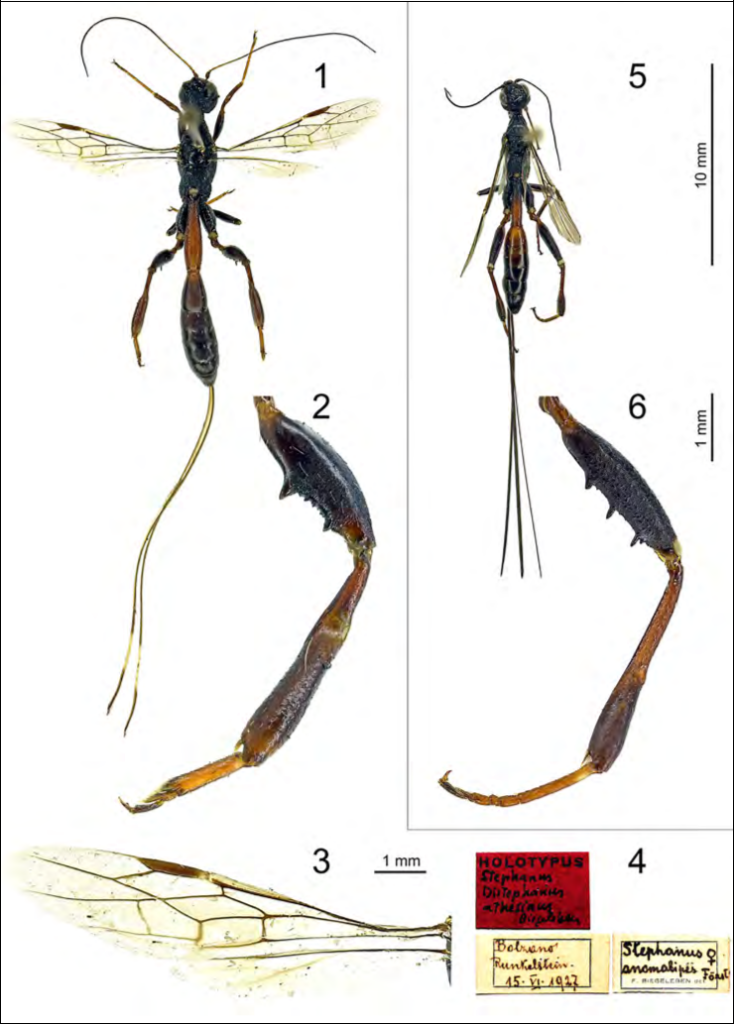
2, hind leg, outer view;
3, wings;
4, original labels.
Figs 5-6. Stephanus serrator (Fabricius, 1798):
5, habitus, dorsal view;
6, hind leg, outer view.
Megischus anomalipes (Förster, 1855) (Figs 1-4, 7)
Stephanus anomalipes Förster, 1855: 228, Holotype♀HNHM (type locality: Budapest, Hungary).
Bothrioceros europaeus Sichel, 1860: 750, 759. Holotype♀MNHN (type locality: Sicily, Italy). Synonymized by Madl (1991).
Megischus europaeus, Sichel, 1865: 484.♀.
Stephanus europaeus, Schletterer, 1889a: 95.♀.
Megischus europaeus, Scaramozzino, 1995: 3.
Stephanus (Distephanus) [sic!] athesinus Biegeleben, 1929: 210-214 (misspell-ing for Diastephanus). Holotype ♀ MSNVE (type locality: Bolzano, Italy). Synonymized by Madl (1991).
Megischus athesinus Scaramozzino, 1995: 3.
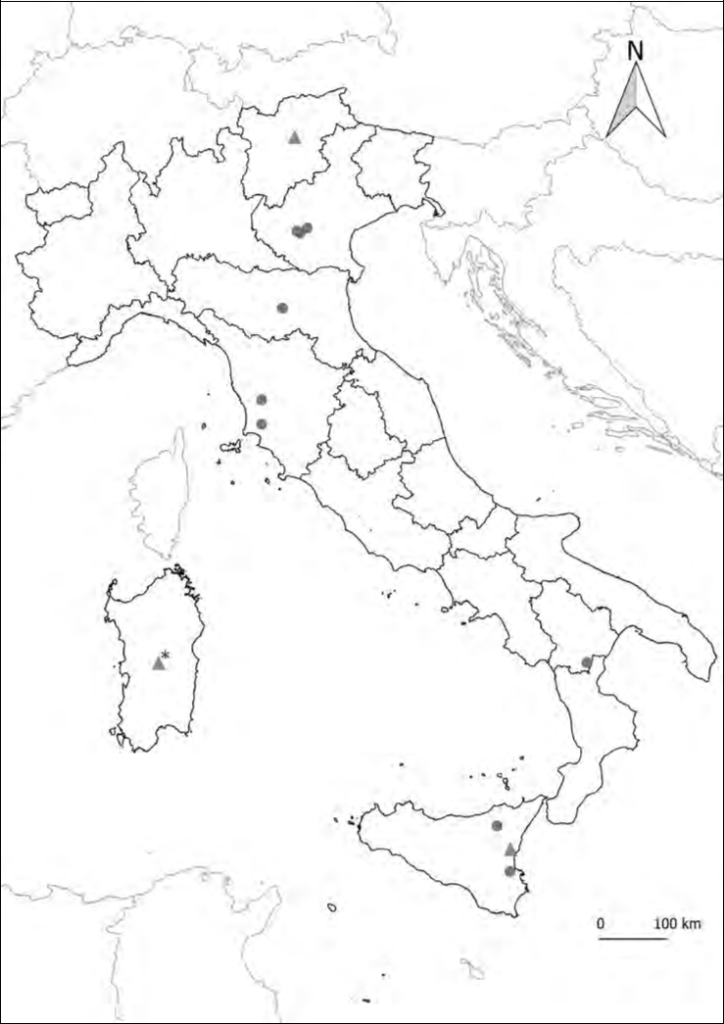
including published localities (green triangles) and new data (red circles); (*) no precise locality given.
Type material of Stephanus (Distephanus) [sic!] athesinus. Holotype♀,Italy:“Bolzano / Runkelstein / 15.vi.1927 // Stephanus / anomalipes Foerster / ♀ / F. BIEGELEBEN det. // HOLOTYPUS / Stephanus / Distephanus / athesinus / Biegeleben” (MSNVE) (Fig. 4).
The specimen is pinned in the mesonotum and it is in excellent condition, with only the last two tarsomeres of the right hindtarsus missing (Fig. 1).
Additional material examined. ITALY.Veneto: 1♀, Vicenza, Colli Berici,Monte del Prete, 4-22.VII.2013, D. Sommaggio leg. Malaise trap (DPDC); 1♀, Vicenza, Colli Berici, Monte Motton, 45°23’05’’N 11°27’21’’E, 23.VII-13. VIII.2013, D. Sommaggio leg. Malaise trap (DPDC); 1♂, same locality and collector, 13.VIII-2.IX.2013, Malaise trap (DPDC); 1♀, Vicenza, Colli Berici, Castegnero, 45°26’42”N 11°34’27”E, 13.VIII-2.IX.2013, D. Sommaggio leg. Malaise trap (DPDC); Tuscany: 1♂, Pisa, Lagoni Rossi, 43°03’49’’N 10°48’05’’E, 24.VII-3.VIII.2011, F. Strumia leg., Malaise trap (TGFC); 1♀, same locality, 3-13.VIII.2011, F. Strumia leg., Malaise trap (TGFC); 1♀, Pisa, Saline di Vol., 43°21’59’’N 10°46’66’’E, 16.VI-2.VII.2009, F. Strumia leg. (TGFC); 1♀, same locality and collector, 9-22.VII.2009 (TGFC); Emilia-Romagna: 1♂, Bologna, Monte San Pietro, San Martino, 27.V.2016, 44.483228°N 11.155754°E L. Scola leg. (DPDC); Basilicata: 2♀, 3♂, Potenza, San Paolo Albanese, Rive torrente Samento, 475 m a.s.l., 40°02’38”N 16°19’14”E, 4.I.2012, ex larva from Spar-tium junceum, emerged 12.VI.2012 (DPDC);Sicily: 2♀, Catania, Etna, BoscoPetrosino, 840 m a.s.l., ex larva from Quercus cerris, emerged during summer 2002, G.F. Turrisi leg. (TGFC); 1♀, 1♂, Catania, Etna: Belpasso, locality Timpazza, 650 m a.s.l., ex larva, from Quercus congesta, emerged 28.VI.2001, G.F. Turrisi leg. (TGFC); 1♀, same locality and collector, emerged 3.VII.2001 (TGFC); 8♀, 8♂, same locality and collector, from wood collected 11.II.2001, emerged summer 2001 (TGFC); 11♀, 4♂, same locality and collector, ex larva, from Quercus congesta, collected 11.II.2002, emerged 15-26.VI.2002 (TGFC); 1♀, Syracuse, Iblei, Carlentini, 400 m a.s.l., 5.V.2006, on dead tree of Quercus suber, G.F. Turrisi leg. (TGFC). GREECE: 1♂, Peloponnesus, Lakonia, 20 kmS Sparti, Chania, 36°54’N 022°30’E, 5-6.V.2012, G. Agnoli & F. Izzillo leg. (DPDC). LEBANON: 1♂, Akkar prov., Fnaideq, 1300 m a.s.l., ex larva from Quercus cerris, emerged 10-30.VI.2000 together with Chlorophorus yachovi, P.Rapuzzi leg. (TGFC); 2♂, Chouf prov., Barouk, 1000 m a.s.l., ex larva emerged from Quercus calliprinos (= Q. coccifera) together with Chlorophorus yachovi, P. Rapuzzi leg. (TGFC); 3♀, 4♂, Jbail, Qartaba, 1300 m a.s.l., ex larva emerged from Quercus sp. together with Chlorophorus yachovi, and Acmaeodera spp., collected 6.VI.1999 and 4-15.V.2000, emerged 18.V.2000, 24.V.2000, 19.VI.2000, 17.VIII.2000, G. Sama leg. (TGFC).
Distribution. South-European with records from Spain, Madeira, France, Italy(including Sardinia and Sicily), Slovakia, Romania, Hungary, former Yugoslavia, Greece (new), and Lebanon (Pagliano, 1986; Turrisi, 2002; van Achterberg, 2002, Madl, 2013).
Redescription of Stephanus (Distephanus) [sic!] athesinus. Holotype♀, bodylength (excluding ovipositor): 15.3 mm; forewing length: 8.1 mm. Colour. Blackish brown; temples, vertex and pronotum dark brown; malar space with a yellowish brown patch; mid and hind coxae and femora dark brown; tarsi and first metasomal tergite reddish-brown; antenna (from second to sixth segments), humeral plate and rest of legs brown; wing weakly infuscate; ovipositor sheath brown, ivory part 0.9 times as long as dark apical part. Head. Antenna with 37 antennomeres, third antennonmere 3.2 times its maximum width, fourth 1.12 times as long as third segment; frons rather convex, rugose-reticulate; three large, anterior, lobe-shaped coronal tooth-like processes, posterior two smaller and lobe-shaped; vertex slightly convex, rugose to transverse-carinate, with a strong carina; occiput transverse-carinate; occipital carina complete, ventrally not reaching base of mandible; temple concave just behind the eyes, then con-vex. Mesosoma. Neck stout, anteriorly concave, postero-dorsally flattened, with two strong complete transverse carinae; pronotal fold lacking; middle part of pronotum with eight weak, irregular transverse carinae; anteromedian carina of pronotum absent, mid part weakly separated from posterior part of pronotum, weakly convex latero-posteriorly; propleuron rugose laterally, without punctures; mesopleuron rugose, with sparse setosity; mesosternum smooth (only scattered punctures present), without setosity; convex part of metapleuron coarsely reticu-late, glabrous; propodeum, axillae, posterior part of mesopleuron and scutellum densely reticulate; scutellar sulcus wide and crenulate. Fore wing with vein 1-M 4.6 times as long as vein 1-SR and 1.1 times vein m-cu; vein 2-RS 1.2 times as long as vein r; vein r 0.2 times length of pterostigma, ending behind level of apex of pterostigma; crossvein between 2+3M and 2Cub present (Fig.). Hind coxa robust, irregularly rugose; hind femur robust, with two strong tooth-like processes, basal process larger than apical one, smaller processes in-between; basal part of hind tibia about one quarter as long as tibia, widened ventrally; hind basitarsus 3-segmented, parallel-sided, its ventral length 5.1 times its width. Metasoma. First tergite 3.8 times as long as its maximum width and 6.6 timesits apical width, ending far behind level of hind coxa and narrowed apically, densely transverse-rugose, smooth in the basal part; second tergite, basally, with curved rugae, largely smooth; rest of second tergite and following tergites micro-sculptured; pygidial area well delimited, surrounded by a setose area; ovipositor sheath 2.3 times as long as forewing and 1.2 times as body length.
Remarks. Madl(1991) synonymized Stephanus (Distephanus) [sic!] athesinus Biegeleben, 1929 under Megischus anomalipes (Förster, 1855), without mention-ing the holotype of Biegeleben. We assume that this synonymy was based solely on the description provided by Biegeleben (1929). Aguiar (2004) suggested that the holotype of Stephanus (Distephanus) [sic!] athesinus, was originally in the private collection of Biegeleben and then probably lost. van Achterberg (2002) redescribed M. anomalipes from a single female specimen from France, without examining the type material. We rediscovered the holotype of Biege-leben (1929) in the collection of the MSNVE, which allowed us to confirm the synonymy with M. anomalipes. Biegeleben (1929) probably identified the specimen under Megischus anomalipes and only subsequently considered it as a new species (Fig. 4). The new species was based on the crossvein between veins 2+3M and 2Cu (Fig. 3) and the small tooth-like process between the basal and the apical robust processes of hind tibiae. According to Aguiar (2004), both characters are variable, thus the differences observed by Biegeleben (1929) are only due to individual variation. Other variations are the body length, which in the holotype is 15.3 mm, whereas 11.2 mm in the specimen examined by van Achterberg (2002) and the antennal segments, which are 37 in the holotype and 32 reported by van Achterberg (2002). Both these two characters perfectly fit with the variation recorded by Madl (1991): up to 16 mm in length and with up to 40 antennomeres. In Italy the species has been recorded only twice from Sicily (Sichel, 1860; Turrisi, 2002) and once from Trentino Alto Adige (Biegeleben, 1929), and there is a record from Sardinia without precise locality (Madl, 2013). We add herein records from additional four regions: Veneto (new), Tuscany (new), Emilia-Romagna (new) and Basilicata (new). The record from Veneto confirms the occurrence of this species in the North-East Italy, 87 years after the previous record (Biegeleben, 1929). The record from Basilicata represents the first finding of this species from South Italy. The current distribution of the species in Italy is summarized in Fig. 7. Additionally, we report the species for the first time from Greece.
Biology. The biology of Megischus anomalipes is poorly known.Sichel(1860)collected a female in September, Biegeleben (1929) a female in June in a oak wood. Turrisi (2002) reported three possible hosts of specimens collected in Sicily, associated with dead wood of Quercus congesta C. Presl. in J. & C. Presl.: Acmaeoderella adspersula (Illiger, 1803), Anthaxia hungarica (Scopoli, 1772) (Bu-prestidae) and Trichoferus fasciculatus (Faldermann, 1837) (Cerambycidae), and two possible hosts of specimens collected in Lebanon associated with Quercus sp.: Chlorophorus yachovi Sama 1996 (Cerambycidae) and Acmaeodera sp. (but probably, more than one species, as indicated in the original label of the specimens from Qartaba) (Buprestidae). The new data highlights the relationships with other Quercus species: Q. coccifera L., Q. cerris L. and Q. suber L., and confirms Chlorophorus yachovi as possible host (see material for detail). The specimenscollected in Basilicata are associated with Spartium junceum L., and those from Veneto were collected in two different sites: a wild meadow at the border of a mixed coniferous and oak wood (Monte Motton) and a mixed wood subjected to anthropogenic cut (Castegnero).
Acknowledgements
We kindly thank the following Italian entomologists: Marco Uliana (the curator of Entomology, Museo di Storia Naturale, Venice) for the loan of the type material and for processing the digital images; Loris Scola (Bologna), Daniele Sommaggio (Vicenza), Guido Pagliano (Torino), Franco Strumia (Pisa), Pierpaolo Rapuzzi (Udine) and Gianfranco Sama (Cesena) for providing useful material. Grateful thanks also go to Lorenzo Munari (Venice) for support and advice.
References
Aguiar A.P. (2004) – World catalog of the Stephanidae (Hymenoptera: Stephanoidea). Zootaxa 753: 120 pp.
Aguiar A.P. (2005) – Stephanoidea Benoit, 1949. Stephanidae Leach, 1815. Version 29 June 2005. http://tolweb.org/Stephanidae/22029/2005.06.29 in The Tree of Life Web Project, http://tolweb.org/ [accessed 15.08.2016]
Aguiar A.P. (2006) – The Stephanidae (Hymenoptera) of Mexico, with description of six new species and key to western Foenatopus Smith. Zootaxa 1186: 1-56.
Aguiar A.P., Jennings J.T. (2005) – First record of Stephanidae (Hymenoptera) from New Caledonia, with descriptions of four new species of Parastephanellus Enderlein. Zootaxa 1001: 1-16.
Aguiar A.P., Jennings J.T. & Turrisi G.F. (2010) – Three new Middle-Eastern species of Foenatopus Smith (Hymenoptera: Stephanidae) with a new host record and key to species with two spots on the metasoma. Zootaxa 2714: 40-58.
Blüthgen P. (1953) – Zur Biologie von Stephanus serrator F. (Hym. Stephanidae). Zo-ologischer Anzeiger 150: 229-234.
Braza R.D. (1989) – Parasitoids of immature sytages of Agrilus sexsignatus (Fisher) (Coleoptera: Buprestidae) attacking Eucalyptus deglupta Blume in Surigao del Sur. The Philippine Entomologist 7: 479-483.
Broad G.R., Quicke D.L.J. (2000) – The adaptive significance of host location by vi-brational sounding in parasitoid wasps. Proceedings of the Royal Society of London B 267:2403-2409.
Brullé A. (1846) – Histoire naturelle des Insectes. Hyménoptères (pp. 536-540). Paris.
Roret., vol. IV, p. I-VIII + 1-680.
Biegeleben B.F. (1929) – Un raro imenottero nuovo nella Venezia Tridentina? Studi Trentini di Scienze Naturali 10(3): 210-214.
Chao H.F. (1964) – Description of new species of Stephanidae (Hymenoptera, Ichneu-monoidea) from South China. Acta entomologica Sinica 13 (3): 376-395.
Förster A. (1855) – Die 2te Centurie neuer Hymenopteren. Verhandlungen des Naturhis-torichen Vereines der preussischen-Rheinlande und Westphalens 12: 226-258.
Hadley A. (2008) – Combine ZM. www.hadleyweb.pwp.blueyonder.co.uk/
Hong C.D., van Achterberg C., Xu Z.F. (2010) – A new species of Megischus Brullé (Hymenoptera, Stephanidae) from China, with a key to the Chinese species. ZooKeys 69: 59-64.
Hong C.D., Xu Z.F. (2011) – A newly recorded genus and species of Family Stephanidae (Hymenoptera, Stephanoidea) from China. Entomotaxonomia 33 (1): 71-73.
LePeletier S.F., Serville J.G. (1825) – In: Latreille, M. Encyclopédie Méthodique. His-toire naturelle. Entomologie, ou histoire des Crustacés, des Arachnides, et des Insectes.Tome 10, part 2: 489 pp.
Madl M. (1991) – Zur Kenntnis der paläarktischen Stephanidae (Hymenoptera, Stepha-noidea). Entomofauna 12 (9): 117-126.
Madl M. (2013) – Fauna Europaea: Stephanidae. In: Mitroiu, M.-D., Fauna Europaea: Hymenoptera, Fauna Europaea version 2.6.2, http://www.faunaeur.org [accessed August 15, 2016].
Masnadi-Yazdinejad A., Lotfalizadeh H. (2009) – Rediscovery of Afromegischus gigas (Schletterer, 1889) (Hymenoptera: Stephanidae) from Iran. North-Western Journal of Zoology 5(1): 8-15.
Pagliano G. (1986) – Aulacidae, Stephanidae ed Evaniidae d’Italia con descrizione di un nuovo Stephanidae del Marocco (Hymenoptera, Ichneumonoidea). Atti del Museo Civico di Storia Naturale di Grosseto 9-10: 1-20.
Rodd N.W. (1951) – Some observations on the biology of Stephanidae and Megalyridae (Hymenoptera). Australian Journal of Zoology 11: 341-346.
Scaramozzino P.L. (1995) – Hymenoptera Trigonalyoidea, Evanioidea, Stephanoidea. In: A. Minelli S. Ruffo, S. La Posta (eds.), “Checklist delle specie della fauna italiana”, 93, Calderini, Bologna.
Schletterer A. (1889) – Monographie der Hymenopteren-Gattung Stephanus Jur.
Berliner Entomologische Zeitschrift 33: 71–160.
Sichel J. (1860) – Liste des Hyménoptères recueillis en Sicile par M. Bellier de la Chavign-erie pendant les mois d’Août à Septembre 1859. Annales de la Société Entomologique de France, 3° série 8: 749-764.
Sichel J. (1865) – Révision des Genres Stephanus Jurine et Megischus Brullé (Famille des Évanides). Annales de la Société Entomologique de France, 4° série 5: 467-487.
Tan J.L., Fan X.L., van Achterberg C., Li T. (2015) – A new species of Pseudomegischus van Achterberg from China, with a key to the species (Hymenoptera, Stephanidae). Zookeys 537: 103-110.
Taylor K.L. (1967) – Parasitism of Sirex noctilio F. by Schlettererius cinctipes (Cresson) (Hymenoptera: Stephanidae). Journal of the Australian Entomological Society 6: 13-19.
Townes H.K. (1949) – The Nearctic species of Stephanidae. Proceeding of the United States National Museum 99: 361-370.
Turrisi G.F. (2002) – Gli Stephanidae di Sicilia, con descrizione del maschio di Megischus anomalipes (Förster, 1855) (Hymenoptera Stephanoidea). Bollettino dell’Accademia Gioenia di Scienze Naturali 35(361): 623-635.
van Achterberg C. (2002) – A revision of the Old World species of Megischus Brullé, Stephanus Jurine and Pseudomegischus gen. nov., with a key to the genera of the fam-ily Stephanidae (Hymenoptera: Stephanoidea). Zoologische Verhandelingen Leiden 339: 204 pp.
van Achterberg C., Yang Z.Q. (2004) – New species of the genera Megischus Brullé and Stephanus Jurine from China (Hymenoptera: Stephanoidea: Stephanidae), with a key to world species of the genus Stephanus. Zoologische Medelingen Leiden: 78(3): 101-117.
van Achterberg C., Quicke D.L.J. (2006) – Taxonomic notes on Old World Stephani-dae (Hymenoptera): description of Parastephanellus matsumotoi sp.n. from Japan, redescription of Commatopus xanthocephalus (Cameron) and keys to the genera Profoenatopus van Achterberg and Megischus Brullé. Tijdschrift voor Entomologie 149: 215-225.
Viereck H.L. (1914) – Type species of the genera of ichneumon flies. Bulletin of the United States National Museum 83: I-IV + 1-186.
Vilhelmsen L., Isidoro N., Romani R., Basibuyuk H.H, Quicke D.L.J. (2001) – Host location and ovipositor in a basal group of parasitic wasps: the subgenual organ, ovipositor apparatus and associated structures in the Orussidae (Hymenoptera, Insecta). Zoomorphology 121: 63-84.
Vilhelmsen L., Turrisi G.F., Beutel R.G. (2008) – Distal leg morphology, subgenual organs and host detection in Stephanidae (Insecta, Hymenoptera). Journal of Natural History 42: 1649-1663.
Visitpanich J. (1994) – The parasitoid wasps of the coffee stem borer, Xylotrechus quad-ripes Chevrolat (Coleoptera, Cerambycidae) in northern Thailand. Japanese Journal of Entomology 62: 597-606.
Völlger E. (1994) – Stephanus serrator (Fabricius, 1798) in Sachsen-Anhalt (Hym., Stephanidae). Entomologische Nachrichten und Berichte 38: 276.

